11 June 2020
Let there be light – to disinfect
Methods of disinfection and hygiene have received a lot of attention over the last months as we all became more aware how important they are to keep us healthy and protect against disease spreading. When talking about antiviral or antibacterial disinfection the first treatments that come to mind are soap, aqueous solutions of alcohols and aqueous solutions containing strongly oxidizing compounds, like peroxides, hypochlorites or chlorine. All of them are effective by different modes of operation, but you have to apply them regularly to achieve continuous protection.
Photodynamic disinfection (PDI) has very different mode of operation and uses daylight or artificial light as an abundant source of energy to eliminate microbes. We need three ingredients to fight bacteria, viruses or other microbes by PDI: Visible light, a dye molecule and air oxygen. Air contains about 21% of molecular oxygen (O2), but a direct excitation is not possible, as the molecule does not absorb any light in or near the visible range. However, the dye molecule absorbs visible light and reaches an electronically excited state, which is very short lived. The encounter of an oxygen molecule with the excited dye molecule, also called sensitizer, leads to a transfer of the excitation energy or a transfer of an electron. Both processes convert molecular oxygen, which is already reactive in its ground state (as we observe it in slow oxidation processes like rusting or bleaching) into super reactive forms of oxygen. One is singlet oxygen, an excited state of molecular oxygen, obtained by energy transfer from the dye molecule; a process named type II mechanism. The corresponding type I mechanism describes an electron transfer from the excited dye molecule to molecular oxygen generating reactive oxygen species (ROS), such as hydroxy radicals or superoxide radical anions.

Overall, the process converts light energy into chemical energy. The extra chemical energy added to molecular oxygen leads to very reactive, but short-lived intermediates. They typically decay in microseconds. This is one of the advantages of the method: Biocide activity of reactive oxygen is only generated when air, light and the sensitizer dye are present. Due its short lifetime, it acts locally, will not diffuse far or accumulate making it a very safe reagent for human use and the environment.
Photodynamic inactivation (PDI) of microbes is not a new discovery. (1) The principle is known since about 130 years and the effect of dyes, air oxygen and light on bacteria, fungi and viruses was investigated in numerous studies and reported in thousands of publications. The high reactivity of the photogenerated biocides and their short lifetimes brings another benefit: Microbes cannot develop resistance as they encounter the reactive oxygen species only once – just before they are destroyed. PDI is therefore active against multiresistance bacteria, which do not respond to antibiotics. (2) On the other hand, will reactive oxygen species not discriminate between “good” or “bad” microbes, but kill them all. A related application is the photodynamic therapy (PDT) in cancer treatment; reactive oxygen species are produced with spatial resolution and their cyctotoxic activity can kill malignant cells. (3)
Among the suitable dye sensitizer molecules for viral PDI are many abundant natural products, such as riboflavin, curcumin, hypericin (key component in St John´s wort) or phytochlorin. Other sensitizer dyes, such as phthalocyanines, carbon materials or phenothiazine derivatives like methylene blue, and classic dye molecules (e.g. Rhodamine B, rose Bengal), offer a wide variety of light absorption and chemical properties required for different applications.
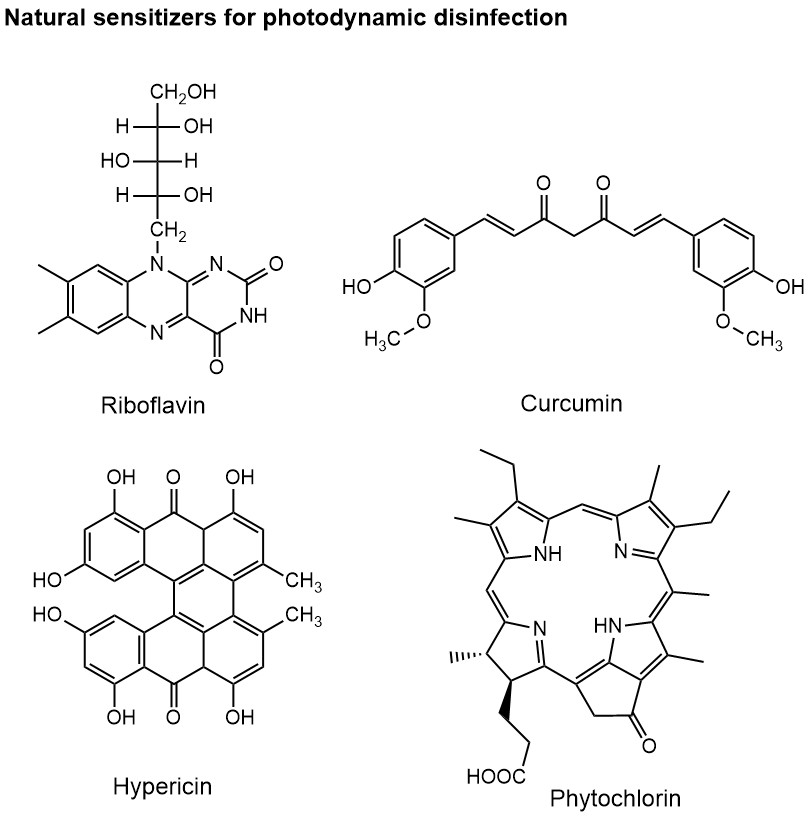
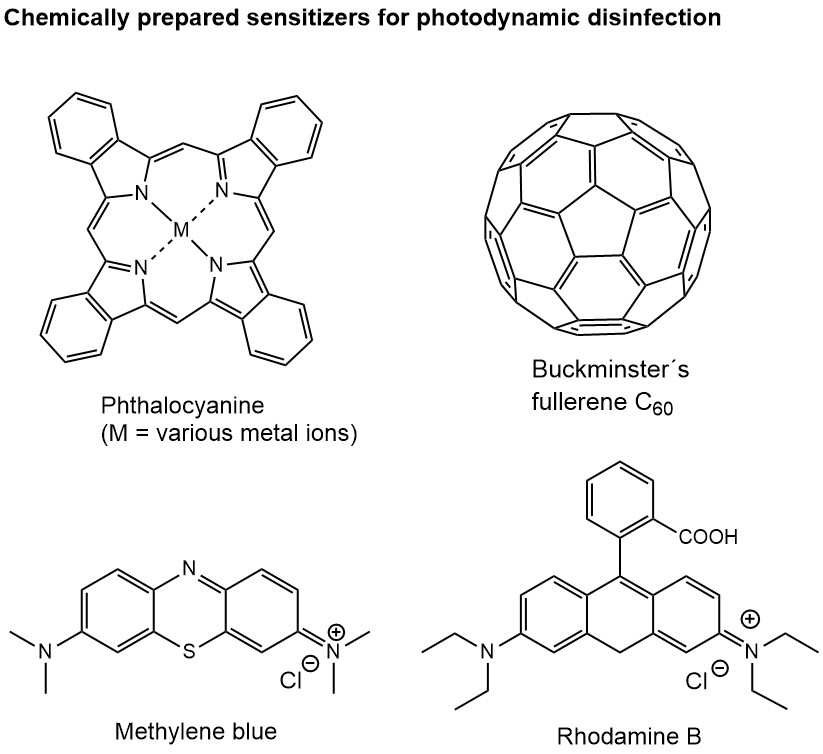
Although the principle and technology of PDI is well established, simple and easy to use, it has not made its broader way into consumer products and everyday life. A small number of applications can be found in dentistry, medical treatments or photocatalytic self-cleaning of surfaces. Would it not be great if surfaces we touch frequently disinfect themselves whenever there is light? They would do this automatically as long as the dye molecules have not completely bleached – and some molecules can be rather stable lasting for days to weeks. Such continuous and daylight driven disinfection is particular welcome in public space, e.g. in busses, commuter trains or shops. Sensitizer dyes can be immobilized on textiles or paper (think of the mask you currently wear frequently) and reduce the level of microbes whenever exposed to daylight. Many other applications of PDI are conceivable improving the level of hygiene in public and private space effectively and with little maintenance.
Let daylight help us to live healthier lives.
References
- Recent review: A. Wiehe, J. M. O’Brien, M. O. Senge Photochem. Photobiol. Sci 2020; doi.org/10.1039/C9PP00211A
- An example using flavin derivatives for the deactivation of multiresistant bacteria: A. Eichner, A. Gollmer, A. Späth, W. Bäumler, J. Regensburger, B. König, T. Maisch, Photochem. Photobiol. Sci. 2015, 14, 387 – 396.
- D. Dolmans, D. Fukumura, R. K. Jain, Nature Reviews Cancer 2003, 3, 380–387; doi.org/10.1038/nrc1071

How does PDI compare to traditional disinfection methods like alcohol-based solutions in terms of efficiency, cost-effectiveness, and environmental impact?
Tel U